Destruction of sodium residues in Lake Lenore (7 photos + 1 video)
At the end of World War II, the US Army had excess sodium metal, which was used to make incendiary bombs. 
The original plan was to sell the excess. When the material was put up for sale, several companies expressed interest. 
But when the metal drums that contained the sodium were inspected, it was discovered that the containers were so damaged that handling and transporting them became extremely dangerous, if not impossible. Sodium reacts violently with water, releasing large amounts of heat and hydrogen gas. 
And this gas often ignites from the heat it releases, causing explosions. Railroad companies refused to transport the material in damaged containers. And the military faced a difficult task: how to dispose of 9,000 tons of reactive sodium? 
The top brass decided to dump the sodium in a lake in Washington. To minimize the environmental impact of dumping thousands of tons of sodium into the water, the Army chose Lake Lenore in Grant County. The choice was made because the water was alkaline and there were no fish. 
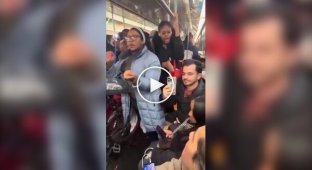
Unbiased newsreel footage released in 1947 shows the military lowering barrel after barrel into the water. The lake was covered in ice. As the barrel began to roll on the ice, machine guns were fired at it. The holes created provided contact with the water. When the chemical reaction began, about 1,500 cubic feet of hydrogen gas were released. The gas ignited, causing a series of spectacular explosions. 
Thus, a once-deadly chemical was transformed into a peacetime pyrotechnic spectacle. 
Lake Lenore is no longer dead. Since the mid-1980s, the state has stocked it with Lahontan cutthroat trout, which thrive in alkaline waters. The species is now the top predator in Lenore and several other alkaline lakes in North America.